|
Process Description - No Solvent
No matter the source of the byproducts, a water soluble anion is extracted
and presented to the organic phase which consists solely of the catalyst and
the organic substrate (mentioned earlier as the alkyl halide
or acyl halide). In general for a continuous process, the reaction is performed
in a Continuous Stirred Tank Reactor (CSTR) operating at the following general
conditions that are determined by the boiling point of the feed organic:
| Temperature: |
10 to 70°C |
| Pressure |
100 to 1000 Kpa (1 - 10 atm) |
| Separations: |
Adiabatic Flash for Volatile Organic Feeds
Distillation for non-Volatile Organics |
| Concentrations: |
Water Soluble component - 0.01% to 25wt%
Organic Soluble component - 0.5% to 100 wt% |
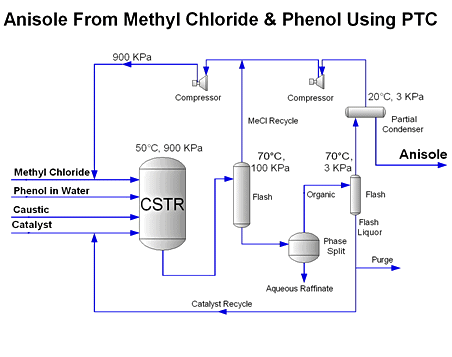
For instance, if methyl chloride (bp = -24°C) is used as the organic feed
material as in the anisole process, the reactor will
operate under pressure and the downstream separations will be flash separators
and partial condensers. If a higher boiling organic compound is used as the
feed organic, like benzyl chloride (bp = 179°C), then the reactor will operate
at atmospheric pressure and the downstream separations will use distillation
and not flash separations. The temperature range of the reaction and the amount
of catalyst used determines the reactor size and operating characteristics.
In all cases an oil-water separation or phase split is performed to recover
organic products and reject the residual aqueous phase that contains halide
(i.e. sodium chloride). The phases can be easily separated in a standard coalescer.
Acyl and Alkyl Halides
- Allyl Chloride
- Allyl Bromide
- Benzyl Chloride
- Benzoyl Chloride
- Ethyl Chloride
- Methyl Bromide
- Methyl Chloride
|








